Supercooled Clouds
Right now the freezer is empty. We see only the light beam shining on the bottom.
We normally expect snow to come from cloudy air, so therefore we need to make
a cloud. There are several ways to make a cloud in this freezer. The easiest
way is the same way that you might make a cloud in front of your face in the
wintertime. Just breathe out! That is what I will do here. I gently blow about
three times to the bottom of the freezer and let the cloudy air mix throughout
the chamber. [If the resulting cloud is too thin or evaporates
quickly, there are some techniques for enhancement.]
The cloud cools instantly to the temperature of the freezer and it will soon
stop moving. But then the cloud will be made of LIQUID droplets that are colder
than 0C or 32F. The droplets should be frozen but are not. They would sparkle
if frozen. We call this SUPERCOOLED water. Supercooled water must be very clean,
which it is, coming as distilled water from my lungs. Supercooled water also
must be warmer than -40. If either condition is violated the water will freeze.
If we do nothing more, this cloud will eventually evaporate. However, there are
several ways in which we can convert this supercooled cloud into ice particles.
One way is to contaminate the cloud with various solids that start the freezing
process. We call these ice nuclei. The best ice nucleus is a yellow-green
chemical called silver iodide. It has a crystal structure nearly identical to
ice and "fools" the water into freezing. In cloud seeding we burn silver iodide
into an invisible smoke. When it gets into a supercooled water cloud at
temperatures between -5C and -20C the silver iodide starts the formation of ice
crystals. I will not use this method in this demonstration. It would certainly
make snow crystals in this freezer. The problem is that I would not be able to
stop the snow to get ready for the next demonstration.
Another method of freezing the droplets is to chill some of the air colder than
-40 degrees. There are several substances that will do this: dry ice, liquid
nitrogen, liquid oxygen, liquid propane.... Most likely, you do not have any
of these around your home. [The exception might be if you have a
CO2 fire extinguisher; they work well. So do empty air rifles (bb
guns).]
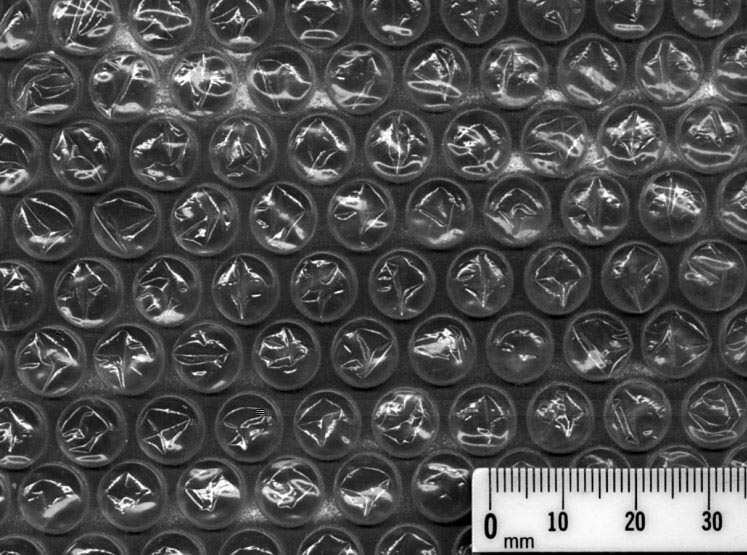
But you probably have some of this...bubble-wrap. I find that the sheets with
small but firm bubbles are best. Large
diameter bubbles are unreliable. Soft bubbles rarely work. Much of the small
bubble wrap on today's market is wimpy, so you have to search carefully for
the firm bubble wrap that works. When I break one of these good bubbles there
is a jet of air coming out of it that rapidly expands. In doing so it apparently
chills itself colder than -40 degrees. It looks like a jet of white smoke comes
out of the bubble. However, there is nothing in the bubbles but air. What looks
like smoke is baby ice crystals. They then move around the freezer, grow, and
eventually sparkle.
When a small bubble is compressed, just before breaking it
shrinks to about one quarter its original size. That means that the air inside
is at about 4 atmospheres pressure. It is the "adiabatic" expansion of that air
that creates the cooling. Large bubbles, wimpy bubbles, and very old plastic
bubble-wrap burst at about 2 atmospheres pressure, which provides insufficient
cooling.
Note: In recent years it appears that bubble wrap has been made intentionally weaker, perhaps to make it decompose more rapidly. Therefore it has become unreliable for this demonstration. That is unfortunate. The bubbles nearly routinely made about 100 million ice crystals in the freezer, just enough for showing the cloud physics in a reasonable time such that three such snow showers could be done in about 20 minutes.
Now I must recommend using dry ice. It can usually be obtained from grocery stores. Get about a pound of it, depending on how much time until the end of the demonstrations and the number of snow showers produced. Break the dry ice into sizes that can be easily held in a gloved hand above the freezer. Use a small pointed tool to knock a very tiny flake of dry ice into the freezer. You should be able to see its smoke trail immediately, or sparkling crystals later moving into the light beam. Getting too much dry ice to fall into the freezer can easily produce a billion crystals. Then there will be too many of them and it takes too long for them to grow as they compete for the water vapor of your breath. That delays when they will be able to fall out to the freezer bottom, allowing a fresh supercooled cloud for the next demonstration. So practice with dry ice to get a minimal amount falling into the freezer.
Back, Next
Home,
sequence,
equipment,
vapor growth,
snowstorm,
crystal growth,
cloud seeding history,
related topics,
water optics,
ice optics,



